Atomic Number Is
The number of protons in a nucleus is called the atomic number, and the number of electrons in orbit around the nucleus is usually equal. Both atoms that have the same number of protons-the atomic number-are also atoms of the same element.

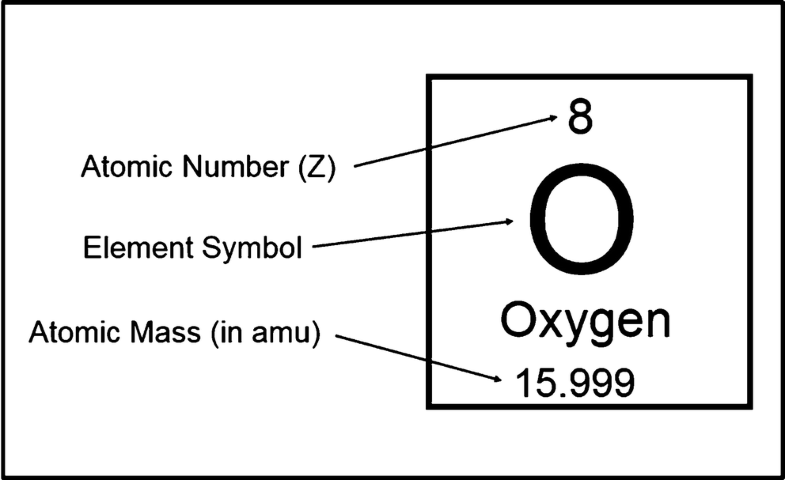
One advantage of using CZT as a detector for high-energy radiation is the high average atomic number of Z = 50, compared with other well-known detector materials such as silicon and germanium with Z = 14 and Z = 32, respectively. Atomic Number of Elements from 1 to 50. List of first 50 elements of the periodic table by atomic number including the chemical symbol and the atomic weight. You can print the list of elements by hitting the print button below. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Fundamental properties of atoms including atomic number. Atomic mass and atomic number may have similarities, but they are used to revealing different characteristics of elements. According to the definitions given, atomic mass which is also called atomic weight is the measured total mass of an element’s atom.
Atomic Number Is 12
Atomic Number Is
Atomic Number, also expressed by the symbol Z, the number of protons in the nucleus of the molecule, and number of electrons in the neutral atom. Atoms with the same number of atoms represent a chemical element. In 1913 H. G. J. Moseley allocated atomic numbers to the elements for the first time. He grouped the elements in an order dependent on some of their X-ray spectra characteristics and then numbered them accordingly. The elements are all grouped to the order of their atomic numbers in the periodic table. The periodic law of Mendeleev was initially based on atomic weights.
